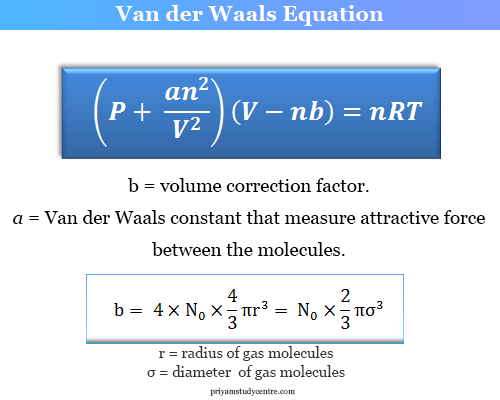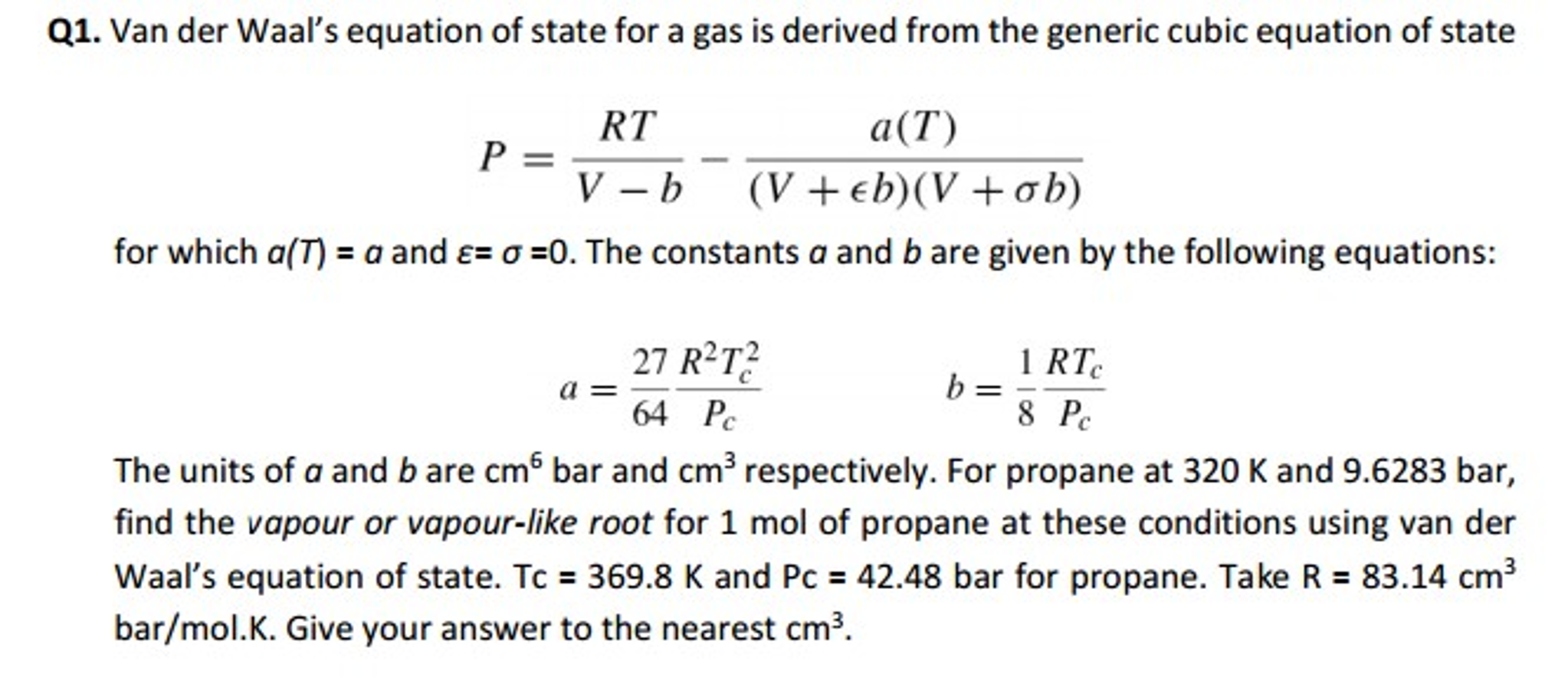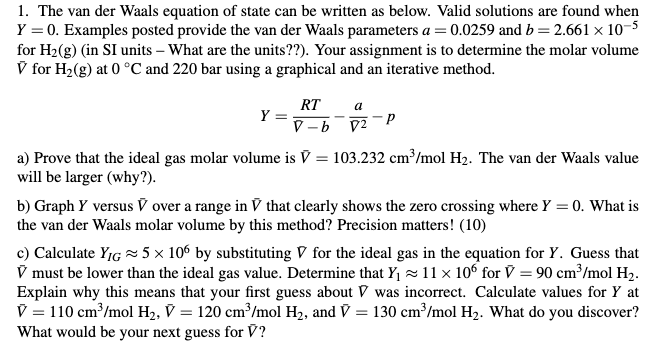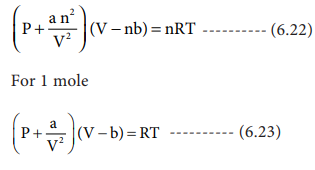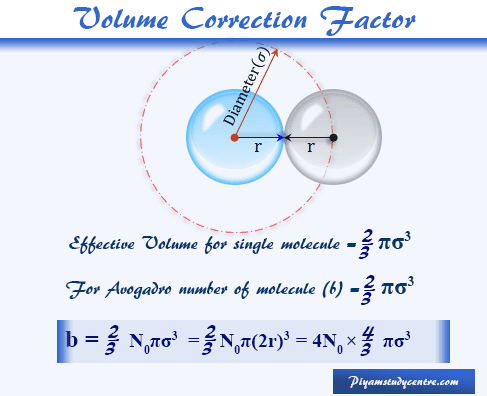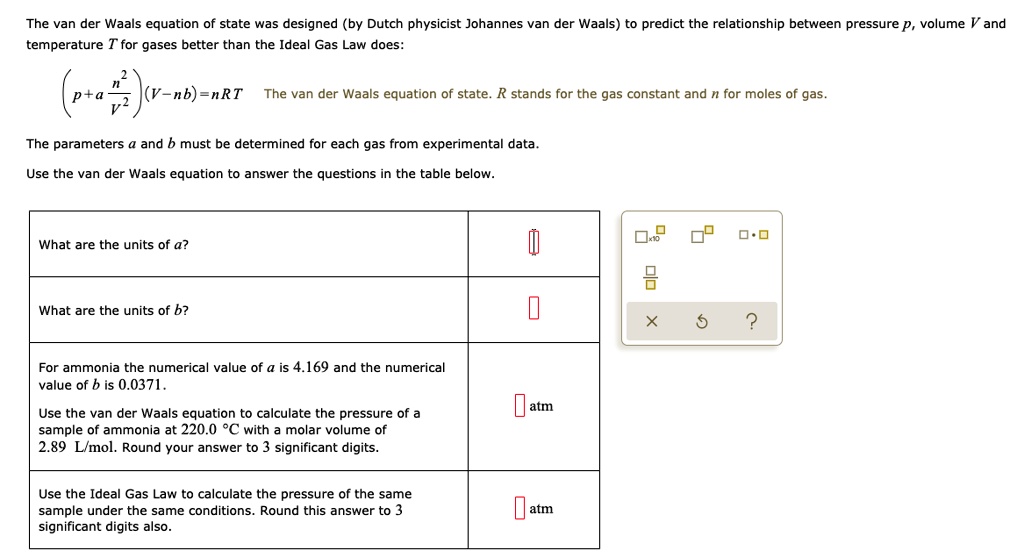
SOLVED: The van der Waals equation of state was designed (by Dutch physicist Johannes van der Waals) to predict the relationship between pressure P, volume V and temperature T for gases better
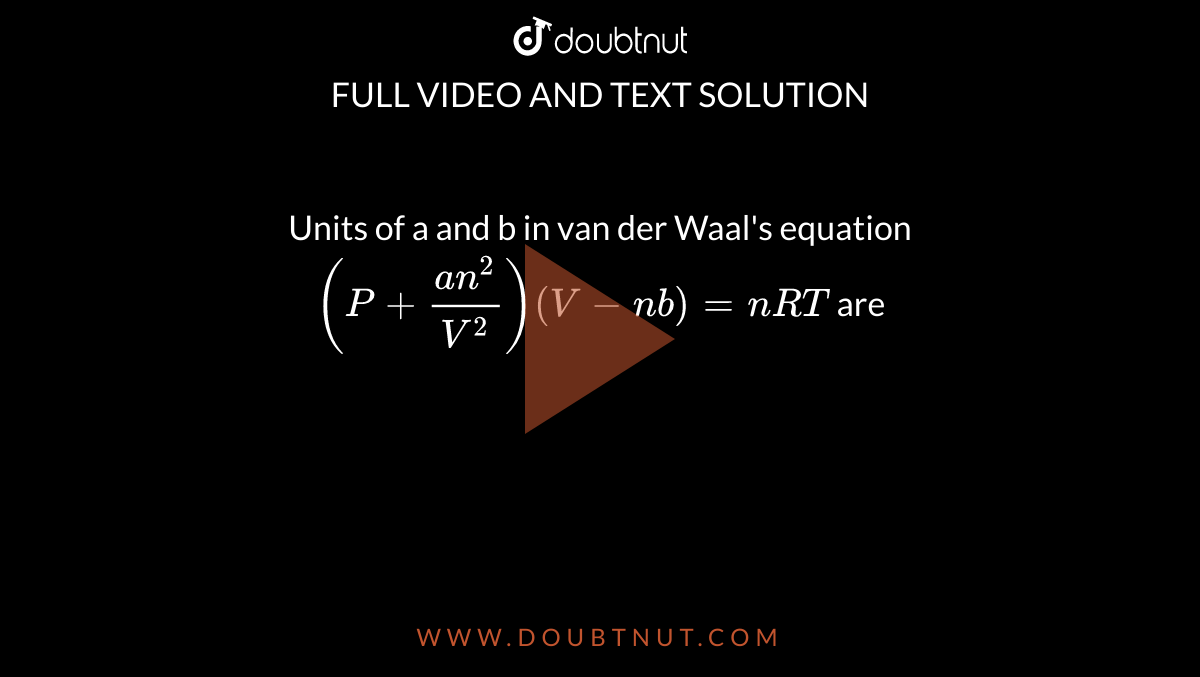
What is the unit of 'a' in terms of fundamental units in Van der waal's equation (P+(a)/(V^(2)))(V-b)=RT?
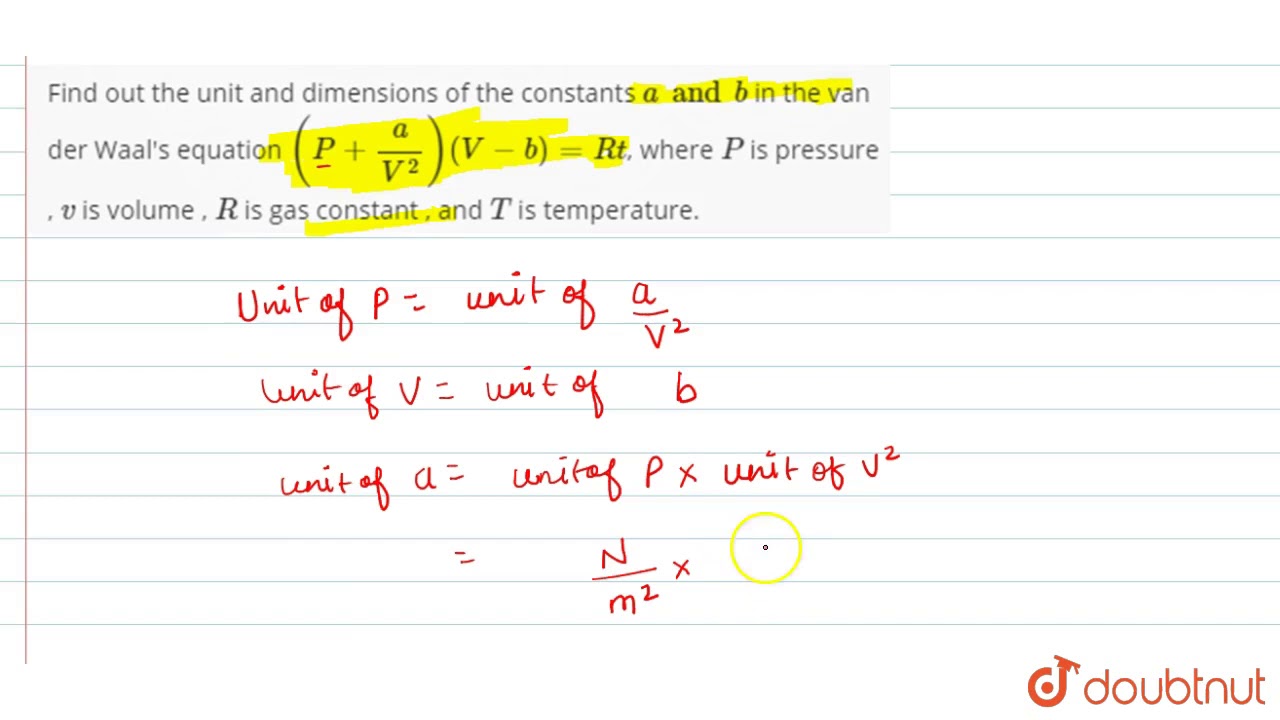
Find out the unit and dimensions of the constants `a and b` in the van der Waal\'s equation `( P... - YouTube
![SOLVED: (a) State the van der Waals gas equation, defining all its terms and their units [3 MARKS] (b) Derive an expression for the excluded volume of a gas (per mole of SOLVED: (a) State the van der Waals gas equation, defining all its terms and their units [3 MARKS] (b) Derive an expression for the excluded volume of a gas (per mole of](https://cdn.numerade.com/ask_images/b85267d5dfb74f3a9fe16fb223ee1147.jpg)
SOLVED: (a) State the van der Waals gas equation, defining all its terms and their units [3 MARKS] (b) Derive an expression for the excluded volume of a gas (per mole of
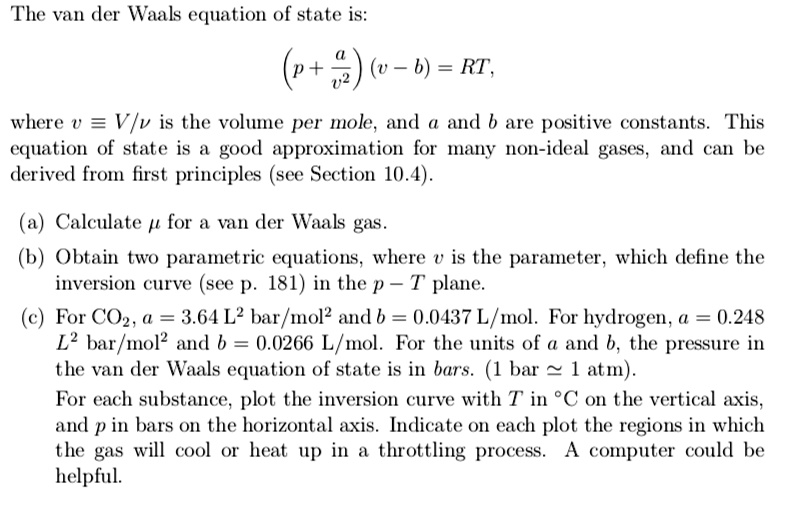
SOLVED: The van der Waals equation of state is: (0- 6) = RT, where v = Vlv is the volume per mole, and and 6 are positive constants. This equation of state