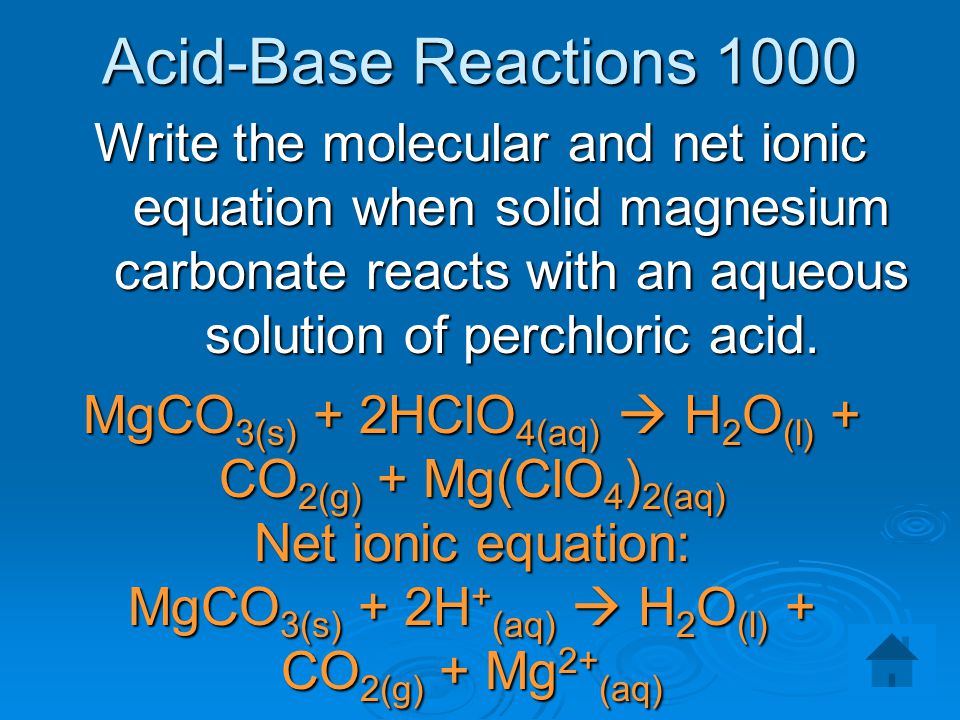
For Your Research. The Four Research Questions 1.What is the chemistry (including an equation) of the process? 2.What are the factors that impact on the. - ppt download

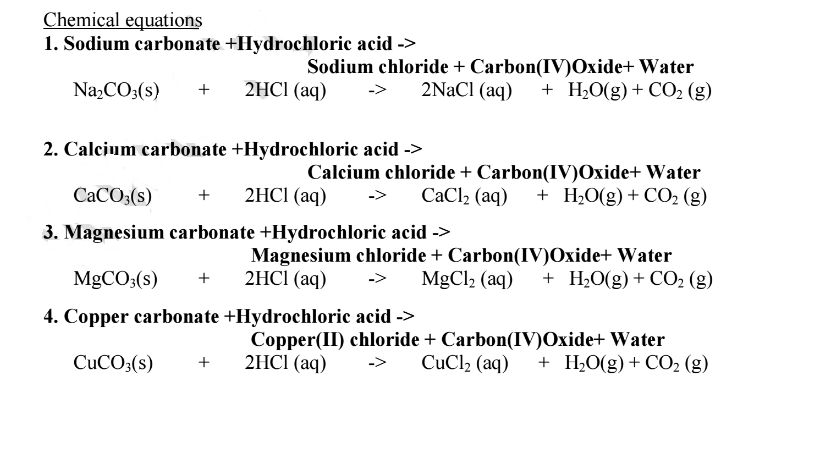
Calcium carbonate reacts with dilute hydrochloric acid according to the equation below - GCSE Science - Marked by Teachers.com
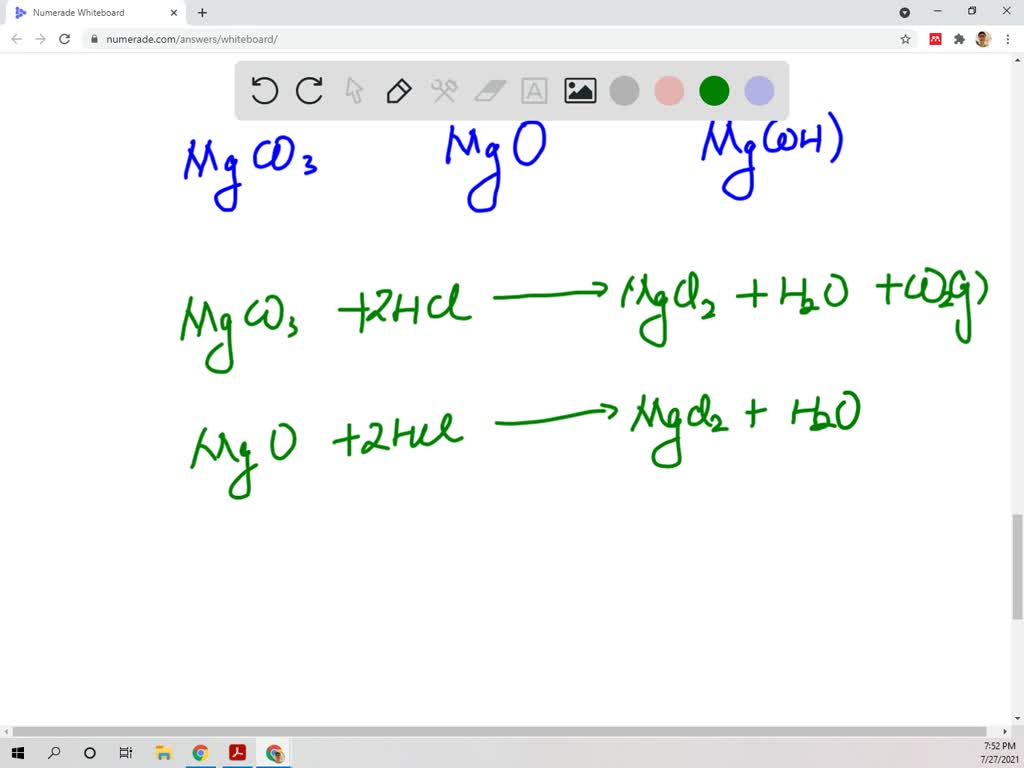
SOLVED: Magnesium carbonate, magnesium oxide, and magnesium hydroxide are all white solids that react with acidic solutions. (a) Write a balanced molecular equation and a netionic equation for the reaction that occurs

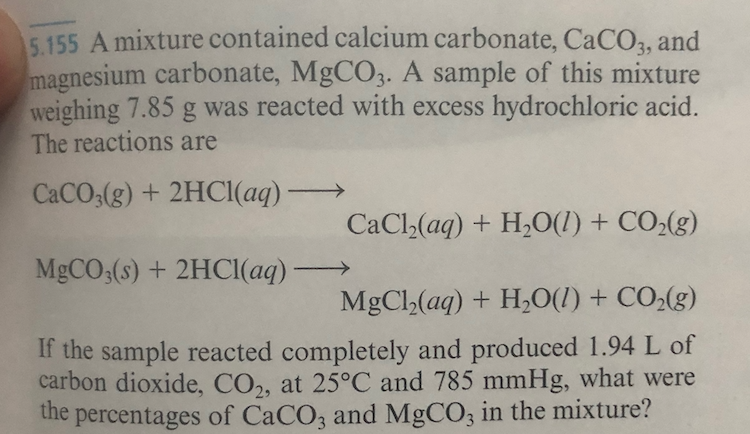
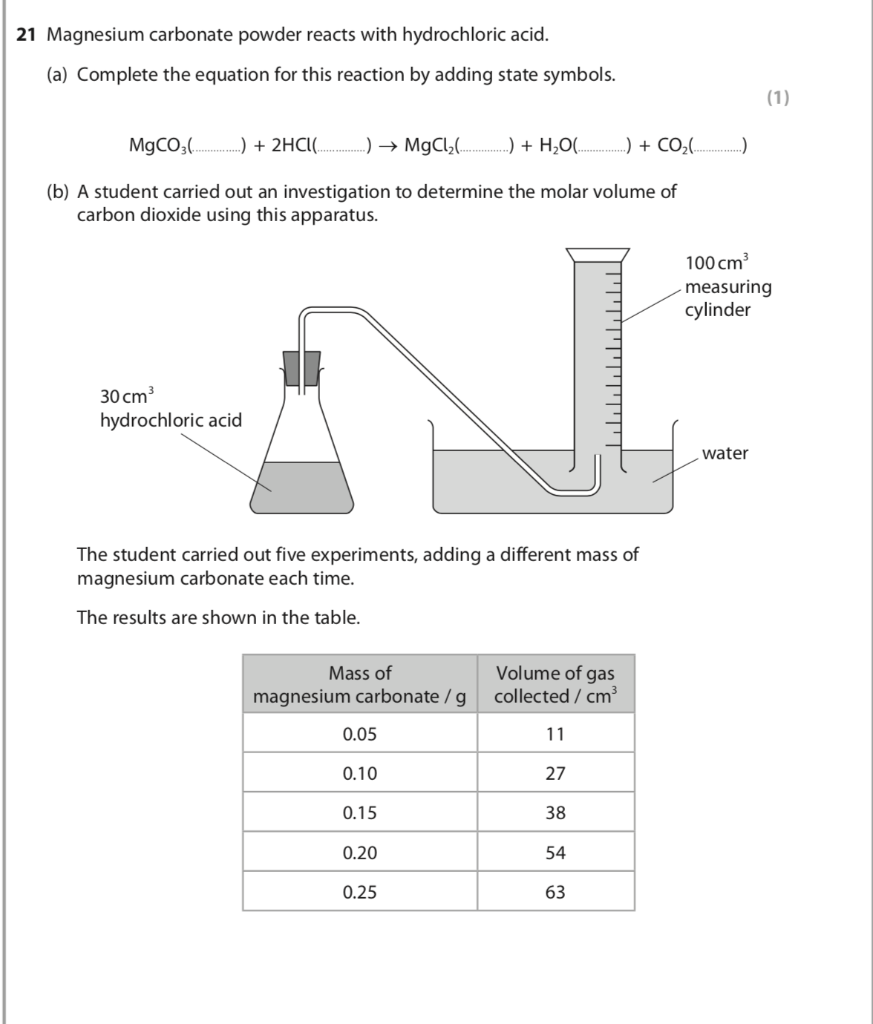
Write the balanced equations for the following reactions, and add the state symbols : (a) Magnesium carbonate reacts with hydrocloric acid to produce magnesium chloride, carbon dioxide and water. (b) Sodium hydroxide
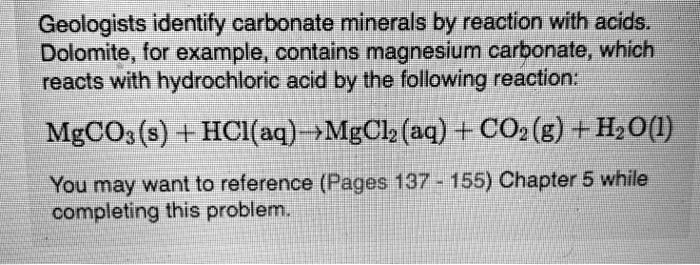
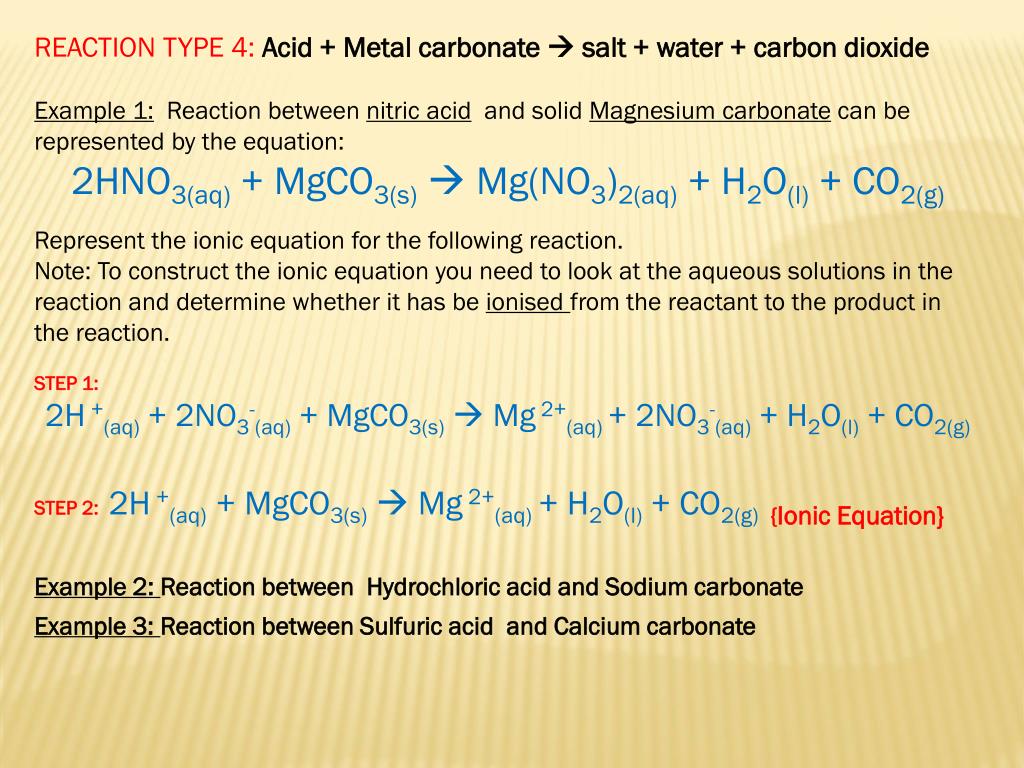
SOLVED: Geologists identify carbonate minerals by reaction with acids Dolomite , for example , contains magnesium carbonate , which reacts with hydrochloric acid by Ihe following reaction: MgCOs (s) + HCl(aq) ,MgClz (